-
PDF
- Split View
-
Views
-
Cite
Cite
Cathy C. Lee, Scott G. Glickman, Donald R. Dengel, Michael D. Brown, Mark A. Supiano, Abdominal Adiposity Assessed by Dual Energy X-Ray Absorptiometry Provides a Sex-Independent Predictor of Insulin Sensitivity in Older Adults, The Journals of Gerontology: Series A, Volume 60, Issue 7, July 2005, Pages 872–877, https://doi.org/10.1093/gerona/60.7.872
Close - Share Icon Share
Abstract
Background. An increase in total adiposity and in particular an abdominal distribution of adiposity may contribute to the decline in metabolic insulin sensitivity observed in older men and women. The objective of this cross-sectional study was to determine which measure of abdominal adiposity would provide the best sex-independent predictor of metabolic insulin sensitivity in older men and women.
Methods. Insulin sensitivity and abdominal adiposity were measured in healthy, nondiabetic older (64 ± 6 years; mean ± standard deviation) men (n = 23) and women (n = 31). Metabolic Insulin Sensitivity Index (SI) was determined from a frequently sampled insulin-assisted intravenous glucose tolerance test. Body fat mass and abdominal fat mass were determined from dual energy X-ray absorptiometry (DXA) scans. Anthropometric measures included waist and hip circumferences, height, and body weight.
Results. Although waist circumference, waist index (waist circumference divided by height), and waist–hip ratio (WHR) were all lower in women than in men, there was no sex difference in DXA L1–L4 fat mass. In univariate analyses, SI was significantly inversely related with body weight, body mass index, waist circumference, waist index, percentage of total body and abdominal fat, and DXA L1–L4 fat mass but not with WHR. The DXA L1–L4 fat mass was identified as the best independent predictor of SI, accounting for 41.2% of the variance (p <.0001) in a stepwise multiple regression model that controlled for sex.
Conclusions. WHR is not associated with SI in either men or women. Abdominal adiposity measured by DXA L1–L4 fat mass provides a sex-independent predictor of SI in older men and women.
AGE-RELATED changes in body composition include an increase in total and abdominal adiposity (1). The increase in adiposity may contribute to the higher prevalence of diabetes mellitus and cardiovascular disease among older individuals (2). In particular, it appears that abdominal adiposity is an important risk factor for these disorders. Abdominal adiposity has, for example, been associated with hypertension (3) and with a decrease in tissue sensitivity to insulin (i.e., insulin resistance) (4). There are sex differences in the amount and distribution of adiposity which need to be considered when the complex interactions between aging, increasing total and abdominal adiposity, insulin sensitivity, and other metabolic disorders are examined. Although women are characterized by lower total body weight, percentage of body fat is significantly greater among women (5). However, in part due to the observation that a simple anthropometric measure, the waist–hip ratio (WHR), is significantly lower in women than in men (1), it has been generally believed that women have lower levels of abdominal adiposity than do men. Therefore, despite higher total percentage of adiposity in women, lower amounts of abdominal adiposity might be expected to attenuate the development of metabolic insulin resistance and other adverse risks that accompany greater adiposity in older women relative to men.
Several methodologic approaches are available to assess the amount and distribution of adipose tissue in humans. Recent criteria for the metabolic syndrome include simple anthropometric measures such as WHR and body mass index (BMI; World Health Organization criteria) and waist circumference (National Centers for Environmental Prediction criteria) (6). Hydrodensitometry has generally been considered the reference standard to determine body composition and total adipose mass, but it does not provide a measure of regional, abdominal adipose tissue distribution. Other techniques include more complex imaging modalities (computerized tomography [CT] scans and magnetic resonance imaging [MRI]). A potential advantage of the imaging methods is the ability to discriminate intra-abdominal from subcutaneous fat, as the metabolic disorders linked to abdominal adiposity are believed to be most directly associated with intra-abdominal visceral fat (4,7–9). Dual energy X-ray absorptiometry (DXA) is another imaging technique to determine body composition that provides measures of lean, adipose, and bone mineral masses. In addition, regional analysis of DXA scans limited to the abdominal area (specifically the region between the top of the first to the bottom of the fourth lumbar vertebrae, L1–L4), has been demonstrated to provide a valid measure of abdominal adiposity in comparison to CT scans (10). Although it is recognized that regional analysis of DXA scans cannot separate intra-abdominal from subcutaneous adiposity, the DXA L1–L4 measure of abdominal adiposity has been demonstrated to be associated with insulin resistance (11,12).
To date, the relationship between DXA L1–L4 fat mass and other measures of total and abdominal adiposity and measures of insulin sensitivity in older men and women has not been examined. The objective of this study was to test the hypothesis that DXA L1–L4 fat mass provides a sex-independent predictor of insulin sensitivity in older humans.
Methods
Participants
Participants were recruited through newspaper advertisement, from the University of Michigan Health System Turner Geriatric Clinic, and from the Human Subject Research Participant Core of the University of Michigan Geriatrics Center. All participants were community dwelling and in good health with stable weight. Study entry criteria included age between 50 and 80 years and BMI between 15 and 40 kg/m2. Individuals received a screening medical history and physical examination prior to their participation. Exclusion criteria were the presence of clinically significant medical illness (including cardiac, renal, hepatic, or gastrointestinal disease) or use of medications that might affect glucose metabolism. A 2-hour, 75 g oral glucose tolerance test was used to confirm the absence of diabetes mellitus (13). Fifty-four nondiabetic men (n = 23) and women (n = 31) provided written informed consent approved by the Institutional Review Board of the University of Michigan prior to their participation.
Individuals being treated with medications (e.g., steroids) that could directly affect glucose metabolism were excluded, and those being treated with antihypertensive medications were tapered from their medications and studied after a minimum of 4 weeks without drug therapy. Studies were performed at the University of Michigan General Clinical Research Center on an outpatient basis beginning at 8 am after an overnight fast. All participants were instructed not to exercise in the 24 hours prior to their study days.
Measurement of Insulin Sensitivity
Insulin sensitivity was assessed during an insulin-assisted frequently sampled intravenous glucose tolerance test (FSIVGTT) (14). Participants were studied in the supine position. Blood samples were obtained from an indwelling retrograde venous catheter placed in a dorsal hand vein 30 minutes prior to study. Arterialized venous samples were obtained by placing this hand into a warming box heated to 60°C (15). An antecubital intravenous catheter was inserted in the contralateral arm to deliver infusions of glucose and insulin. Three resting blood samples taken immediately prior to glucose administration were collected to determine fasting plasma insulin and glucose levels. A bolus injection of 50% glucose (0.3 g/kg of body weight) was administered as an intravenous push over 30 seconds, followed by blood sampling at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 35, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180 minutes after injection. At 20 minutes, an intravenous bolus of 0.02 U of regular insulin per kilogram of body weight was administered.
Measurements of Body Composition
Total and abdominal body composition were assessed by DXA (Lunar DPX-IQ 240 densitometer, medium collimation, medium speed; Lunar Radiation Corp., Madison, WI) within 2 weeks of the FSIVGTT. Analysis of the total body scan was performed using Extended Research Mode. Quality assurance tests were run every morning using a standard block of tissue-equivalent material. The coefficient of variation (CV) for the DXA is ≤2.0%.
Anthropometric assessment included measurement of waist (minimum circumference of the waist) and hip (maximum circumference of the buttocks) circumferences. Triplicate circumference measurements of the natural waist (minimum frontal plane diameter and umbilicus) were taken using a Gullick handled woven tape (16). Skin fold thicknesses were measured three times at the suprailiac, suprailium, and abdomen with a Harpenden skin fold caliper following standardized procedures, except for the abdomen skin fold where a vertical rather than horizontal fold was measured (17). The average value of the three trials was used as the criterion value. A single technician performed all of the skin fold and circumference measures.
Participants did not eat, drink, urinate, defecate, or exercise between tests. Participants were clothed in either T-shirt and athletic shorts or a standard hospital gown. Body weight was measured to the nearest 0.1 kg using a medical beam scale. The scale was zeroed before each test and was calibrated on a weekly basis. Height was measured to the nearest 0.5 cm using a stadiometer.
Analytical Methods and Statistical Analysis
Blood samples for plasma glucose and insulin were collected into chilled glass tubes containing sodium heparin, stored on ice, and separated immediately following each study. Plasma was stored at −70°C until assay. Plasma glucose was measured by the autoanalyzer glucose oxidase method, and plasma insulin by double antibody radioimmunoassay (coefficient of variation = 5%) in the Core Laboratory of the University of Michigan Diabetes Research and Training Center. All samples from a given participant were analyzed in the same assay run.
The Insulin Sensitivity Index (SI) was calculated from a least-squares fitting of the temporal pattern of glucose and insulin throughout the FSIVGTT using the MINMOD program (Copyright R. N. Bergman, 1986) (14). SI is a measure of the effect of an increment in plasma insulin to enhance the fractional disappearance of glucose. Area under the curve for glucose and insulin values during the oral glucose tolerance tests were calculated using the trapezoid rule in GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, CA).
Analysis of DXA scans used Lunar software version 4.5c (extended research analysis). The percentage of total body fat was determined from the total fat mass divided by the body weight. The DXA measure of abdominal adiposity (DXA L1–L4) was determined with the manual analysis component of the Lunar software package. A rectangle was drawn on the digital scan image bounded superiorly by the horizontal line identifying the T12/L1 intervertebral space, inferiorly by the horizontal line denoting the L4/L5 intervertebral space, and bilaterally by connecting the two horizontal lines in a region free of tissue. Abdominal adiposity, DXA L1–L4, was measured as the fat mass within this region. The percentage of abdominal adiposity was determined from the DXA L1–L4 fat mass divided by total body fat mass. Prior work from our research group has shown this approach to have good interrater reliability, reproducibility, and validity in comparison to multislice CT scans (18). Additional calculated measures of body composition included BMI (weight divided by height squared), WHR (waist circumference divided by hip circumference), and waist index (waist circumference divided by height).
Sex differences in participant characteristics, glucose and insulin levels, SI, and measures of body composition were evaluated using t tests. The relationships between SI and body composition measures were assessed by multiple regression analysis that included a sex factor. A stepwise multiple regression model was developed to determine which body composition variables were independent predictors of SI among the participants; this model incorporated a forced sex variable.
Data analysis was conducted using StatView (version 5.0; SAS Institute, Chapel Hill, NC), SAS version 6.12 (SAS Institute, Inc., Cary, NC), and GraphPad Prism version 3.00 for Windows (GraphPad Software). A Bonferroni correction was used to account for the four major hypotheses tested so that a level of p ≤.01 was used to indicate statistical significance. Data are expressed as means ± standard error.
Results
Anthropometric, body composition, and metabolic characteristics of the study population subdivided by sex are provided in Table 1. The men and women in the present study were of similar age. However, men were significantly taller and heavier than were women, although there was no sex difference noted in BMI. Waist circumference, WHR, and waist index were all greater in men than in women, although the sex difference in waist index was not statistically significant. Total body fat mass determined from the DXA scan tended to be greater in women, and, given their lower body weight, percentage of body fat was significantly greater in women than in men. Although percentage of abdominal fat was significantly greater in men than in women, there was no sex difference in DXA L1–L4 fat mass. Fasting glucose levels were significantly higher in men than in women. There was no sex difference noted in fasting insulin levels, the 2-hour oral glucose tolerance test glucose values, area under the curve for glucose or insulin, or SI.
Univariate simple regression coefficients unadjusted for sex between SI and the anthropometric and body composition independent variables are provided in the correlation matrix presented in Table 2. SI was inversely correlated with each of these measures except WHR (all p ≤.01). The strongest univariate relationship with SI was the DXA L1–L4 mass. As expected, there were significant positive correlations noted between the majority of the anthropometric and body composition measures. However, percentage of body fat was not significantly related to waist circumference, weight, or percentage of abdominal fat. There was no significant relationship found between SI and age.
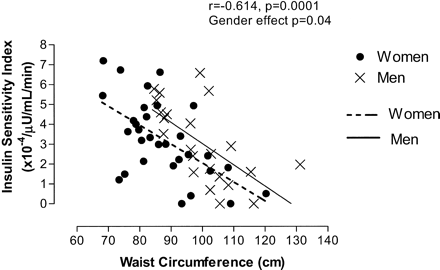
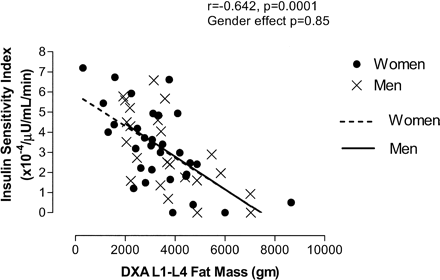
The effect of sex on the relationship between SI and anthropometric and body composition variables was evaluated by multiple regression analysis including a sex grouping variable. As stated above, WHR was not significantly associated with SI (r = −0.231, p =.25). There was also no significant sex effect identified in the relationship between WHR and SI (p =.36), and there were significant sex effects identified in the relationships between SI and waist circumference (r = −0.614, p =.0001, sex effect p =.04) (Figure 1) and between SI and percentage of body fat (r = −0.548, p =.0001, sex effect p =.003). Only the relationship between SI and DXA L1–L4 had no significant sex effect identified (r = −0.642, p =.0001, sex effect p =.85) (Figure 2). No significant interactions were found between WHR, waist circumference, percentage of body fat, or DXA L1–L4 and sex. Tests for collinearity were run because of the significant intercorrelations between many of these measures using variance inflation factor values, condition indices, and a design matrix. Variance inflation factor values < 10 (range 3.44–8.86), condition indices < 100, and condition number for a design matrix equal to 7.75 indicate the absence of significant collinearity.
Finally, to determine which of the anthropometric and body composition variables would independently predict SI, stepwise multiple regression analysis was performed. This analysis incorporated a forced sex variable and five independent body composition variables: BMI, percentage of body fat, waist index, DXA L1–L4 fat mass, and percentage of abdominal fat. DXA L1–L4 fat mass was the only independent predictor of SI that entered this model; it accounted for 41% of the population variance in SI. Another stepwise regression model was developed and limited to the measures of abdominal adiposity (waist index, DXA L1–L4 fat mass, and percentage of abdominal fat) to determine which of these would be the best independent predictor of SI. The result of this analysis was identical to that of the full model with DXA L1–L4 fat mass being the only variable that entered the model. If these analyses were performed without taking sex into account, DXA L1–L4 fat mass remained the best independent predictor of SI.
Discussion
In this older, nondiabetic participant population, DXA L1–L4 fat mass provides the best sex-independent predictor of SI. All of the anthropometric and DXA measures of total and abdominal adiposity were inversely and significantly related to SI except WHR (Table 2). In contrast, WHR, which is commonly used as an indicator of abdominal adiposity, was not significantly related to SI whether or not the analysis was adjusted for sex. The strongest of the univariate relationships was noted between SI and DXA L1–L4 fat mass. There were no sex differences identified in SI, fasting insulin level, age, BMI, or hip circumference. As expected, women were shorter, had lower body weight and higher percentage of body fat, and smaller waist circumference and WHR compared with men. The sex difference in these anthropometric measures of total and abdominal adiposity would suggest that women have lower levels of abdominal adiposity. However, there was no significant sex difference identified in waist index, DXA L1–L4 fat mass, or percentage of abdominal fat. The relationship between SI and DXA L1–L4 fat mass remained the strongest predictor in a multivariate regression model that incorporated a sex variable. Moreover, there was not a significant sex effect noted for this relationship. Other measures of total and abdominal adiposity did not enter into this model. We conclude from these results that DXA L1–L4 fat mass provides a sex-independent predictor of insulin sensitivity in older humans. In addition, WHR and waist circumference appear to underestimate the degree of abdominal adiposity in older women which was found to be similar to that of older men.
A number of studies (8,9,11,12,19–30) have identified that various measures of total and abdominal (or upper body) adiposity are related to insulin sensitivity in older participant populations. Prior to the availability of imaging modalities, WHR was commonly used to reflect the extent of abdominal adiposity (1). However, among the anthropometric indices of abdominal adiposity, measures of waist circumference (30–32) or waist index (33) have been found to be better predictors of insulin sensitivity. These findings are consistent with the observation that waist index was closely related to intra-abdominal fat area determined from CT scans in both men and women (33). In the current study, no significant relationship was identified between WHR and insulin sensitivity, whereas SI was negatively related to both waist circumference and waist index.
Although there is an age-related increase in WHR in men and women, WHR remains lower in women suggesting that older women have less abdominal adiposity than do older men (1). Among the studies that included men and women to relate adiposity to insulin sensitivity (8,22,28,30,31), only the study by Cefalu and colleagues (8) incorporated an analysis of the effect of sex on the relationship between the amount and distribution of adiposity and insulin sensitivity. The focus of their study was to isolate the effects of aging from that of visceral abdominal adiposity determined from cross-sectional fat area by CT scan to the SI from an FSIVGTT. Accordingly, the study population included a wider age range (23–83 years). Among this population, the independent predictors of SI were intra-abdominal fat and WHR; there were no significant effects noted for age or sex after these two measures were included in a regression model. However, given that there were only three male participants over the age of 60 years in their sample, it is likely that the age and sex distribution of this participant population was not optimal to detect an effect of sex on the relationship between SI and intra-abdominal adiposity among older individuals. The participant populations for other studies that have addressed this relationship include only males (11,20,24–26,29) or only females (9,12,19,21,23,27). Thus, the present report is the first to specifically examine the influence of sex on the relationship between measures of total and abdominal adiposity derived from regional analysis of DXA scans and insulin sensitivity in older humans.
The results from the current study suggest that older men and women have similar levels of abdominal fat mass measured from regional analysis of DXA scans. The results from several other studies that have used either CT- or MRI-derived measures of intra-abdominal adiposity conducted in younger participant samples have shown that there is no sex difference in intra-abdominal fat area, but that subcutaneous abdominal fat area is greater in women than in men (8,22,28,30). Because the DXA measure of abdominal fat mass cannot discriminate between subcutaneous and visceral fat, additional studies using a measure of total abdominal adipose volume (subdivided by intra-abdominal and subcutaneous) will be required to confirm the lack of sex difference in intra-abdominal adiposity among older healthy participants suggested from the DXA L1–L4 fat mass results.
Previous studies (10,18) have been performed to validate the use of DXA L1–L4 fat mass to represent abdominal adiposity as determined from three-dimensional imaging modalities. Our study was not designed to directly compare the relative strength of the relationship between insulin sensitivity and the DXA-derived measure of abdominal adiposity to that obtained from one of the three-dimensional imaging systems. A frequently cited limitation of the two-dimensional DXA regional analysis of abdominal adiposity in comparison to the CT- or MRI-derived abdominal fat areas is its inability to separate intra-abdominal from subcutaneous adiposity. Despite this potential limitation, in this participant population the DXA L1–L4 fat mass proved to be a significant, sex-independent predictor of insulin sensitivity. Indeed, there remains some degree of debate concerning which component of abdominal fat (intra-abdominal or subcutaneous) confers the stronger association with adverse metabolic risk factors, including insulin sensitivity. Although the general observation is that intra-abdominal fat mass is the more important component in this respect (8,34,35), some reports have demonstrated that subcutaneous abdominal fat is a unique and independent predictor of insulin sensitivity (22,23,28,29).
We acknowledge several potential limitations inherent in our study. First, we recognize that using DXA to measure body composition and central obesity does not differentiate between subcutaneous and visceral fat. Second, we did not measure other variables in all participants such as aerobic capacity or blood pressure that are known to be associated with insulin resistance. Therefore, we are unable to estimate the variance in SI that might be explained by these other measures relative to the contribution of abdominal adiposity.
Conclusion
Compared to WHR, waist circumference, or waist index, the DXA L1–L4 fat mass is the best sex-independent predictor of insulin sensitivity in an older, nondiabetic population. In addition, abdominal adiposity as measured by the DXA L1–L4 fat mass was found to be similar in older men and women. Therefore, the other methods of measuring abdominal adiposity (WHR and waist circumference) significantly underestimate the degree of abdominal adiposity in older women.
Decision Editor: John E. Morley, MB, BCh
Association between waist circumference and Insulin Sensitivity Index (SI) by sex
Association between DXA L1–L4 fat mass and Insulin Sensitivity Index (SI) by sex
Participant Characteristics.
| Characteristic . | Men (N = 23) . | Women (N = 31) . | p Value . | |||
|---|---|---|---|---|---|---|
| Age, y | 63.5 ± 1.1 | 64.8 ± 1.3 | .46 | |||
| Anthropometric | ||||||
| Height, cm | 174.7 ± 1.0 | 162.8 ± 1.3 | .0001 | |||
| Weight, kg | 87.1 ± 3.2 | 72.8 ± 2.8 | .0015 | |||
| Body mass index, kg/m2 | 28.5 ± 0.9 | 27.4 ± 0.9 | .41 | |||
| Waist, cm | 99.8 ± 2.4 | 87.4 ± 2.2 | .0005 | |||
| Hip, cm | 107.4 ± 2.0 | 107.4 ± 2.1 | .99 | |||
| Waist–hip ratio | 0.93 ± 0.01 | 0.81 ± 0.01 | <.0001 | |||
| Waist index | 0.57 ± 0.01 | 0.54 ± 0.01 | .07 | |||
| DXA | ||||||
| Total body fat mass, kg | 26.6 ± 2.1 | 30.5 ± 1.9 | .18 | |||
| Percentage of body fat, % | 29.9 ± 1.4 | 40.7 ± 1.4 | <.0001 | |||
| L1–L4 fat mass, kg | 3.7 ± 0.3 | 3.3 ± 0.3 | .43 | |||
| Percentage of abdominal fat, % | 13.7 ± 0.3 | 10.5 ± 0.4 | <.0001 | |||
| Metabolic | ||||||
| Fasting glucose, mg/dl | 102 ± 2 | 93 ± 1 | <.0001 | |||
| Fasting insulin, μU/ml | 13.3 ± 1.6 | 11.7 ± 1.3 | .44 | |||
| SI [×104/(μU/ml/min)] | 3.1 ± 0.4 | 3.2 ± 0.4 | .72 | |||
| 2-h glucose, mg/dl | 131 ± 6 | 132 ± 7 | .86 | |||
| Glucose AUC, mg/ml/min | 18,213 ± 641 | 17,259 ± 606 | .29 | |||
| Insulin AUC, μU/ml/min | 8865 ± 889 | 10,022 ± 1067 | .43 | |||
| Characteristic . | Men (N = 23) . | Women (N = 31) . | p Value . | |||
|---|---|---|---|---|---|---|
| Age, y | 63.5 ± 1.1 | 64.8 ± 1.3 | .46 | |||
| Anthropometric | ||||||
| Height, cm | 174.7 ± 1.0 | 162.8 ± 1.3 | .0001 | |||
| Weight, kg | 87.1 ± 3.2 | 72.8 ± 2.8 | .0015 | |||
| Body mass index, kg/m2 | 28.5 ± 0.9 | 27.4 ± 0.9 | .41 | |||
| Waist, cm | 99.8 ± 2.4 | 87.4 ± 2.2 | .0005 | |||
| Hip, cm | 107.4 ± 2.0 | 107.4 ± 2.1 | .99 | |||
| Waist–hip ratio | 0.93 ± 0.01 | 0.81 ± 0.01 | <.0001 | |||
| Waist index | 0.57 ± 0.01 | 0.54 ± 0.01 | .07 | |||
| DXA | ||||||
| Total body fat mass, kg | 26.6 ± 2.1 | 30.5 ± 1.9 | .18 | |||
| Percentage of body fat, % | 29.9 ± 1.4 | 40.7 ± 1.4 | <.0001 | |||
| L1–L4 fat mass, kg | 3.7 ± 0.3 | 3.3 ± 0.3 | .43 | |||
| Percentage of abdominal fat, % | 13.7 ± 0.3 | 10.5 ± 0.4 | <.0001 | |||
| Metabolic | ||||||
| Fasting glucose, mg/dl | 102 ± 2 | 93 ± 1 | <.0001 | |||
| Fasting insulin, μU/ml | 13.3 ± 1.6 | 11.7 ± 1.3 | .44 | |||
| SI [×104/(μU/ml/min)] | 3.1 ± 0.4 | 3.2 ± 0.4 | .72 | |||
| 2-h glucose, mg/dl | 131 ± 6 | 132 ± 7 | .86 | |||
| Glucose AUC, mg/ml/min | 18,213 ± 641 | 17,259 ± 606 | .29 | |||
| Insulin AUC, μU/ml/min | 8865 ± 889 | 10,022 ± 1067 | .43 | |||
Notes: DXA = Dual energy X-ray absorptiometry; SI = Insulin Sensitivity Index; AUC = area under the curve.
Participant Characteristics.
| Characteristic . | Men (N = 23) . | Women (N = 31) . | p Value . | |||
|---|---|---|---|---|---|---|
| Age, y | 63.5 ± 1.1 | 64.8 ± 1.3 | .46 | |||
| Anthropometric | ||||||
| Height, cm | 174.7 ± 1.0 | 162.8 ± 1.3 | .0001 | |||
| Weight, kg | 87.1 ± 3.2 | 72.8 ± 2.8 | .0015 | |||
| Body mass index, kg/m2 | 28.5 ± 0.9 | 27.4 ± 0.9 | .41 | |||
| Waist, cm | 99.8 ± 2.4 | 87.4 ± 2.2 | .0005 | |||
| Hip, cm | 107.4 ± 2.0 | 107.4 ± 2.1 | .99 | |||
| Waist–hip ratio | 0.93 ± 0.01 | 0.81 ± 0.01 | <.0001 | |||
| Waist index | 0.57 ± 0.01 | 0.54 ± 0.01 | .07 | |||
| DXA | ||||||
| Total body fat mass, kg | 26.6 ± 2.1 | 30.5 ± 1.9 | .18 | |||
| Percentage of body fat, % | 29.9 ± 1.4 | 40.7 ± 1.4 | <.0001 | |||
| L1–L4 fat mass, kg | 3.7 ± 0.3 | 3.3 ± 0.3 | .43 | |||
| Percentage of abdominal fat, % | 13.7 ± 0.3 | 10.5 ± 0.4 | <.0001 | |||
| Metabolic | ||||||
| Fasting glucose, mg/dl | 102 ± 2 | 93 ± 1 | <.0001 | |||
| Fasting insulin, μU/ml | 13.3 ± 1.6 | 11.7 ± 1.3 | .44 | |||
| SI [×104/(μU/ml/min)] | 3.1 ± 0.4 | 3.2 ± 0.4 | .72 | |||
| 2-h glucose, mg/dl | 131 ± 6 | 132 ± 7 | .86 | |||
| Glucose AUC, mg/ml/min | 18,213 ± 641 | 17,259 ± 606 | .29 | |||
| Insulin AUC, μU/ml/min | 8865 ± 889 | 10,022 ± 1067 | .43 | |||
| Characteristic . | Men (N = 23) . | Women (N = 31) . | p Value . | |||
|---|---|---|---|---|---|---|
| Age, y | 63.5 ± 1.1 | 64.8 ± 1.3 | .46 | |||
| Anthropometric | ||||||
| Height, cm | 174.7 ± 1.0 | 162.8 ± 1.3 | .0001 | |||
| Weight, kg | 87.1 ± 3.2 | 72.8 ± 2.8 | .0015 | |||
| Body mass index, kg/m2 | 28.5 ± 0.9 | 27.4 ± 0.9 | .41 | |||
| Waist, cm | 99.8 ± 2.4 | 87.4 ± 2.2 | .0005 | |||
| Hip, cm | 107.4 ± 2.0 | 107.4 ± 2.1 | .99 | |||
| Waist–hip ratio | 0.93 ± 0.01 | 0.81 ± 0.01 | <.0001 | |||
| Waist index | 0.57 ± 0.01 | 0.54 ± 0.01 | .07 | |||
| DXA | ||||||
| Total body fat mass, kg | 26.6 ± 2.1 | 30.5 ± 1.9 | .18 | |||
| Percentage of body fat, % | 29.9 ± 1.4 | 40.7 ± 1.4 | <.0001 | |||
| L1–L4 fat mass, kg | 3.7 ± 0.3 | 3.3 ± 0.3 | .43 | |||
| Percentage of abdominal fat, % | 13.7 ± 0.3 | 10.5 ± 0.4 | <.0001 | |||
| Metabolic | ||||||
| Fasting glucose, mg/dl | 102 ± 2 | 93 ± 1 | <.0001 | |||
| Fasting insulin, μU/ml | 13.3 ± 1.6 | 11.7 ± 1.3 | .44 | |||
| SI [×104/(μU/ml/min)] | 3.1 ± 0.4 | 3.2 ± 0.4 | .72 | |||
| 2-h glucose, mg/dl | 131 ± 6 | 132 ± 7 | .86 | |||
| Glucose AUC, mg/ml/min | 18,213 ± 641 | 17,259 ± 606 | .29 | |||
| Insulin AUC, μU/ml/min | 8865 ± 889 | 10,022 ± 1067 | .43 | |||
Notes: DXA = Dual energy X-ray absorptiometry; SI = Insulin Sensitivity Index; AUC = area under the curve.
Correlation Matrix Between Dependent Variable Insulin Sensitivity Index (SI) and Independent Body Composition Variables.
| Correlation Matrix Variables . | Weight . | BMI . | Waist . | WHR . | Waist Index . | Percentage of Body Fat . | DXA L1–L4 . | Percentage of Abdominal Fat . |
|---|---|---|---|---|---|---|---|---|
| SI [×104/(μU/ml/min)] | −.563 | −.633 | −.567 | −.195 | −.603 | −.406 | −.642 | −.366 |
| Weight, kg | .876 | .907 | .537 | .792 | .258 | .860 | .587 | |
| BMI, kg/m2 | .819 | .339 | .873 | .518 | .874 | .420 | ||
| Waist, cm | .718 | .938 | .170 | .803 | .642 | |||
| Waist–hip ratio | .602 | −.345 | .342 | .669 | ||||
| Waist index | .322 | .791 | .521 | |||||
| Percentage of body fat, % | .557 | −.147 | ||||||
| DXA L1–L4, g | .579 |
| Correlation Matrix Variables . | Weight . | BMI . | Waist . | WHR . | Waist Index . | Percentage of Body Fat . | DXA L1–L4 . | Percentage of Abdominal Fat . |
|---|---|---|---|---|---|---|---|---|
| SI [×104/(μU/ml/min)] | −.563 | −.633 | −.567 | −.195 | −.603 | −.406 | −.642 | −.366 |
| Weight, kg | .876 | .907 | .537 | .792 | .258 | .860 | .587 | |
| BMI, kg/m2 | .819 | .339 | .873 | .518 | .874 | .420 | ||
| Waist, cm | .718 | .938 | .170 | .803 | .642 | |||
| Waist–hip ratio | .602 | −.345 | .342 | .669 | ||||
| Waist index | .322 | .791 | .521 | |||||
| Percentage of body fat, % | .557 | −.147 | ||||||
| DXA L1–L4, g | .579 |
Notes: All correlations in bold are significant with p ≤.01.
BMI = body mass index; WHR = waist–hip ratio; DXA = dual energy X-ray absorptiometry.
Correlation Matrix Between Dependent Variable Insulin Sensitivity Index (SI) and Independent Body Composition Variables.
| Correlation Matrix Variables . | Weight . | BMI . | Waist . | WHR . | Waist Index . | Percentage of Body Fat . | DXA L1–L4 . | Percentage of Abdominal Fat . |
|---|---|---|---|---|---|---|---|---|
| SI [×104/(μU/ml/min)] | −.563 | −.633 | −.567 | −.195 | −.603 | −.406 | −.642 | −.366 |
| Weight, kg | .876 | .907 | .537 | .792 | .258 | .860 | .587 | |
| BMI, kg/m2 | .819 | .339 | .873 | .518 | .874 | .420 | ||
| Waist, cm | .718 | .938 | .170 | .803 | .642 | |||
| Waist–hip ratio | .602 | −.345 | .342 | .669 | ||||
| Waist index | .322 | .791 | .521 | |||||
| Percentage of body fat, % | .557 | −.147 | ||||||
| DXA L1–L4, g | .579 |
| Correlation Matrix Variables . | Weight . | BMI . | Waist . | WHR . | Waist Index . | Percentage of Body Fat . | DXA L1–L4 . | Percentage of Abdominal Fat . |
|---|---|---|---|---|---|---|---|---|
| SI [×104/(μU/ml/min)] | −.563 | −.633 | −.567 | −.195 | −.603 | −.406 | −.642 | −.366 |
| Weight, kg | .876 | .907 | .537 | .792 | .258 | .860 | .587 | |
| BMI, kg/m2 | .819 | .339 | .873 | .518 | .874 | .420 | ||
| Waist, cm | .718 | .938 | .170 | .803 | .642 | |||
| Waist–hip ratio | .602 | −.345 | .342 | .669 | ||||
| Waist index | .322 | .791 | .521 | |||||
| Percentage of body fat, % | .557 | −.147 | ||||||
| DXA L1–L4, g | .579 |
Notes: All correlations in bold are significant with p ≤.01.
BMI = body mass index; WHR = waist–hip ratio; DXA = dual energy X-ray absorptiometry.
This work was supported in part by the General Clinical Research Center (National Institutes of Health grant RR-00042), by the Department of Veterans Affairs Medical Research Service and Ann Arbor Geriatric Research Education and Clinical Center, by a VA Research Career Development Award, by the Claude D. Pepper Older Americans Center at the University of Michigan (AG 08808), by the Michigan Diabetes Research and Training Center (National Institute of Diabetes & Digestive & Kidney Diseases grant P60DK2572), and by National Institute on Aging grant K23 AG00924.
We thank Becky Olson, Amy Woznick, and Marla Smith for their technical assistance and Andrzej Galecki, MD, PhD for his statistical advice.
Portions of this work were presented at the Central Society for Clinical Research and the American Geriatrics Society national meeting in 1997.
References
Shimokata H, Tobin JD, Muller DC, Elahi D, Coon PJ, Andres R. Studies in the distribution of body fat: I. Effects of age, sex, and obesity.
Kohrt WM. Abdominal obesity and associated cardiovascular comorbidities in the elderly.
Meyers DA, Goldberg AP, Bleecker ML, Coon PJ, Drinkwater DT, Bleecker ER. Relationship of obesity and physical fitness to cardiopulmonary and metabolic function in healthy older men.
Bonora E, Del Prato S, Bonadonna RC, et al. Total body fat content and fat topography are associated differently with in vivo glucose metabolism in nonobese and obese nondiabetic women.
Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study.
Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic Syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the Metabolic Syndrome in a prospective cohort study.
Yamashita S, Nakamura T, Shimomura I, et al. Insulin resistance and body fat distribution: contribution of visceral fat accumulation to the development of insulin resistance and atherosclerosis.
Cefalu WT, Wang ZQ, Werbel S, et al. Contribution of visceral fat mass to the insulin resistance of aging.
Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM.
Svendsen OL, Hassager C, Bergmann I, Christiansen C. Measurement of abdominal and intra-abdominal fat in postmenopausal women by dual energy x-ray absorptiometry and anthropometry: comparison with computerized tomography.
Paradisi G, Smith L, Burtner C, et al. Dual energy x-ray absorptiometry assessment of fat mass distribution and its association with the insulin resistance syndrome.
Rissanen P, Hämäläinen P, Vanninen E, Tenbunen-Eskelinen M, Uusitupa M. Relationship of metabolic variables to abdominal adiposity measured by different anthropometric measurements and dual-energy x-ray absorptiometry in obese middle-aged women.
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus.
Bergman RN. Toward physiological understanding of glucose tolerance: minimal model approach.
Forster HV, Dempsey J, Thompson E, Virduk E, Dopico GA. Estimation of arterial pO2, pCO2, pH, and lactate from arterialized venous samples.
Behnke AR, Wilmore JH. Evaluation and Regulation of Body Build and Composition. Englewood Cliffs, NJ: Prentice Hall; 1974.
Lohman TG, Roche AF, Martorell R, eds. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics; 1988.
Glickman SG, Marn CS, Supiano MA, Dengel DR. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity.
Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women.
Furler SM, Poynten AM, Kriketos AD, et al. Independent influences of central fat and skeletal muscle lipids on insulin sensitivity.
Rendell M, Hulthén UL, Törnquist C, Groop L, Mattiasson I. Relationship between abdominal fat compartments and glucose and lipid metabolism in early postmenopausal women.
Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance.
Sites CK, Calles-Escandón J, Brochu M, Butterfield M, Ashikaga T, Poehlman ET. Relation of regional fat distribution to insulin sensitivity in postmenopausal women.
Sparrow D, Borkan GA, Gerzof SG, Wisniewski C, Silbert CK. Relationship to fat distribution to glucose tolerance: results of computed tomography in male participants of the normative aging study.
Coon PJ, Rogus EM, Drinkwater D, Muller DC, Goldberg AP. Role of body fat distribution in the decline in insulin sensitivity and glucose tolerance with age.
Boden G, Chen X, DeSantis RA, Kendrick Z. Effects of age and body fat on insulin resistance in healthy men.
Marcus MA, Murphy L, Pi-Sunyer FX, Albu JB. Insulin sensitivity and serum triglyceride level in obese white and black women: relationship to visceral and truncal subcutaneous fat.
Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat.
Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men.
Pouliot MC, Després JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women.
Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity.
Iwao S, Iwao N, Muller DC, Elahi D, Shimokata H, Andres R. Effect of aging on the relationship between multiple risk factors and waist circumference.
Ashwell M, Cole TJ, Dixon AK. Ratio of waist circumference to height is strong predictor of intra-abdominal fat.
Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity.