-
PDF
- Split View
-
Views
-
Cite
Cite
Craig J. Galbán, Stefan Maderwald, Frank Stock, Mark E. Ladd, Age-Related Changes in Skeletal Muscle as Detected by Diffusion Tensor Magnetic Resonance Imaging, The Journals of Gerontology: Series A, Volume 62, Issue 4, April 2007, Pages 453–458, https://doi.org/10.1093/gerona/62.4.453
Close - Share Icon Share
Abstract
Background. Loss of muscle mass and strength is a common symptom in the elderly population. This is partly a result of the structural changes that occur during the aging process. We applied diffusion tensor magnetic resonance imaging (DTMRI) to determine if water diffusivity in skeletal muscle changes with age.
Methods. Thirty-eight healthy men, ranging from 27 to 67 years of age, were recruited for this study. The total population was grouped by age (Young < mean age 46.4: n = 20; Old ≥ mean age 46.4: n = 18) and body mass index (BMI; Normal < 25: n = 20; Over ≥25: n = 18). The principal, second, and third eigenvalues (λ1 ≥ λ2 ≥ λ3), and fractional anisotropy (FA), were calculated from the diffusion tensor of the soleus, lateral and medial gastrocnemius, and anterior tibialis (AT). Analyses included comparison of groups and linear regressions.
Results. The older adults showed a significant reduction in the eigenvalues of the plantar flexors (∼7%, p <.05) and FA of AT (∼10%, p <.05) from that of the younger adults. No age differences were observed in the FA of the plantar flexors or eigenvalues of AT. λ1 and λ2 had a linear dependence on age in the plantar flexors, whereas AT showed age dependence in λ3 and FA.
Conclusions. We demonstrated that DTMRI is sensitive to age-related changes in muscle, and that the effects of aging differ between the plantar flexors and AT.
ADVANCED age is commonly accompanied by a loss in muscle mass and strength in healthy adults (1–6). This process is referred to as sarcopenia (7,8). Age-related muscle wasting leads to a higher incidence of falls and fractures, and subsequently a reduction in the quality of life (9–12). Due to the increasing longevity of individuals and the expanding elderly population, the progression of muscle dysfunction during aging is a topic of scientific and clinical significance.
Age-related muscle wasting is partly due to the structural changes that occur in skeletal muscle (13–17). Atrophy of muscle mass of up to 50% and higher has been observed in aged muscle tissue (1,3,15,18–21). A contributing factor to this finding is a reduction in muscle fiber size and number. The former has been shown to be disproportionate between the principal fiber types: Types I and II (18,21). Type II fibers, which are highly anaerobic, tend to have a marked decrease in size during aging. In contrast, Type I fibers, which are highly aerobic, retain their size. Fiber grouping is also a common age-related abnormality (18). Generally, fibers of different types have a “mosaic” appearance in young muscle tissue. In elderly muscle, fibers of the same type tend to group resulting in a “patchy” appearance. This finding has been attributed to incomplete activation of muscle tissue as a result of improper innervation (22,23). Another common structural alteration is deformation at the fiber level (18). Adjacent fibers in healthy young muscle have similar cross-sectional geometries. As a result of aging, muscle fibers begin to deform, some appearing round and others more elongated. These structural changes extend beyond a fiber's cross-section and include fiber architecture. Morse and colleagues (24) observed that the pennation angle of the soleus (SOL) in older adults was 18% smaller than that in young adults. In the medial gastrocnemius (MG), the fiber length was 15% smaller. It has also been reported that nonmuscle tissue—intramuscular fat and connective tissue—increase as a function of age (25). These combined changes contribute in large part to the loss of strength observed in muscle.
Experimental techniques capable of measuring at the microscopic level would be advantageous in studying sarcopenia. Diffusion tensor magnetic resonance imaging (DTMRI) has been used to indirectly study microscopic changes in various tissues (26–30). The theoretic basis of this technique is that, during random diffusion, a molecule probes its environment (31,32). Conceptually, diffusion can be visualized with ellipsoids where the eigenvectors of the DT represent the three orthogonal axes of an ellipsoid with the lengths represented by the DT eigenvalues. In skeletal muscle, it has been shown that the principal eigenvalue (λ1) corresponds to diffusion directed along the fibers' length (26,33,34), leaving the other two eigenvalues (λ2 and λ3) in the cross-sectional plane. Skeletal muscle also has three well-defined eigenvalues, such that λ1 > λ2 > λ3. Differences in the eigenvalues are assumed to be a result of the complex structural hierarchy of muscle, where the underlying secondary structures have yet to be determined.
In this study, we sought to extend our previous work (35,36) by determining whether DTMRI is sensitive to age-related changes in muscle tissue. We also investigated, using linear regression techniques, the relationship between age and DTMRI-acquired parameters from specific muscle groups and whether these parameters demonstrate similar trends to literature results of sarcopenia (37). Experiments were performed on a healthy population of men ranging from 27 to 67 years of age. DTMRI was used to measure water diffusivity and tissue anisotropy in the SOL, anterior tibialis (AT), and lateral gastrocnemius (LG), and MG. Effects of height, weight, and body mass index (BMI) on DTMRI parameters were also analyzed.
Materials and Methods
Thirty-eight healthy men, ages ranging from 27–67 years (mean ± standard deviation [SD], 46.4 ± 14.1 years), volunteered to participate in this study, which was approved by the local Ethics Committee Review Board at the University Hospital of Essen. At the time of the study, only six participants were involved in any strength or endurance training. These men were undergoing training for a marathon at the time of the experiment. Their ages were 39, 52, 53, 56, 58, and 67. Height and weight were measured for all individuals and were used to calculate the BMI (ratio of weight [kg] to squared height [m2]). This index is considered a simple anthropometric feature of fatness (38).
MRI Experimental Protocol
MRI examinations were performed with the participants' feet first supine in a 1.5 T full-body scanner (Siemens Sonata, Erlangen, Germany). A standard transmit/receive extremity coil was used to scan the calf region of each participant's left leg. To reduce overall examination time, only one leg was scanned. We assumed that our measurements would be independent of leg dominance. This assumption was based on reported observations of similar muscle strength and function in the lower extremities of healthy men (39,40). Participant ankles were not fixed to any angle because muscle fiber lengths have a negligible effect on diffusion measurements (35,41). T2-weighted axial images were acquired using a single-shot spin-echo EPI sequence with the following parameters: repetition time (TR)/echo time (TE) = 2000/95 ms, field of view (FOV) = 18 × 13.5 cm2, interpolated matrix = 256 × 192 (acquired matrix = 128 × 96), slice thickness = 6 mm, and number of excitations (NEX) = 16 (total scan time = 4 minutes). Diffusion gradients were applied along six directions with a b value of 400 s/mm2. A T2-weighted image, used for calculating the DT, was acquired with a b value of 0 s/mm2. A single slice was obtained at the thickest cross-sectional area of the calf. Placement of this image provided ample visualization of all muscle groups being studied. To specify the regions of interest (ROI) for the individual muscles and the thickest cross-sectional area of the calf, a high-contrast axial T1-weighted Fast Low Angle Shot (FLASH) sequence was used with the following parameters: TR/TE = 165/4.5 ms, NEX = 2, 36 slices over the entire calf, and using the same FOV, matrix, and slice thickness as the T2-weighted images (total scan time = 55 seconds). Slice positioning for the subsequent T2-weighted images was determined by manually selecting the T1-weighted image with the thickest cross-sectional area of the calf.
DTMRI Data Analysis
T2-weighted images were processed, and the DT was calculated for each pixel as described by Basser and Pierpaoli (42). As discussed in our previous work, monoexponential behavior in diffusion and T2 were assumed for this study (35,36). ROIs were specified for four different muscles by using the individual FLASH image. The muscles analyzed were the SOL, LG, MG, and AT. The ROIs included approximately 300 pixels. For each ROI, four calculations were made: three eigenvalues of the DT (λ1 ≥ λ2 ≥ λ3) and the fractional anisotropy (FA), which is a function of all three eigenvalues that varies from 0 (isotropic) to 1 (anisotropic) (31). These quantities were averaged over all the pixels within the ROI. Fascia, nerves, blood vessels, and fat were excluded by manual segmentation from the ROI before calculation of the four parameters. All computations were performed using MATLAB 7.0 (The MathWorks, Inc., Natick, MA).
Data and Statistical Analyses
The means of the eigenvalues and FA were calculated for each muscle ROI. DTMRI parameters, age, height, weight, and BMI were then averaged for each age or BMI group. All results were presented as the mean ± standard error of the mean (SEM). The data were analyzed either by separating the total population by age or BMI, or as continuous variables. Age cutoff was based on the mean age of the participants. All participants < 46.4 years (n = 20: 34.1 ± 1.2 years) were categorized as Young and ≥46.4 years as Old (n = 18: 60.1 ± 1.2 years). This criterion was chosen due to the lack of a clear partition in the total population. The BMI cutoff was set at 25 kg/m2, where participants with a BMI < 25 kg/m2 were designated Normal (n = 20: 23.0 ± 0.4 kg/m2) and ≥25 kg/m2 as Over (n = 18: 28.3 ± 0.8 kg/m2) (43). The six athletes had normal BMI. A one-way analysis of variance (ANOVA) was performed between the individual groups for age and BMI. Relationships between the DTMRI parameters and age, weight, height, or BMI were determined using simple linear regressions. Multiple linear regressions were performed to evaluate the age effect on the DTMRI parameters when controlling for weight, height, and BMI. Statistical significance was set to p <.05. All statistical tests were performed using SPSS 13.0 (SPSS Inc., Chicago, IL).
Results
Group Comparisons
Young participants were found to be significantly taller than old participants (181.9 ± 1.6 cm vs 174.8 ± 1.6 cm; p =.004), whereas participants with normal BMI weighed less than those with an elevated BMI (73.2 ± 2.1 kg vs 90.5 ± 1.9 kg; p <.0001).
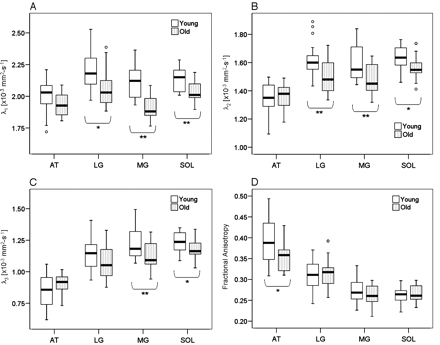
Age-related differences in the eigenvalues are presented in Figure 1, A–C, for each muscle group. Statistical variations were observed in all three eigenvalues of SOL and MG, λ1 and λ2 of LG, and none for AT. All eigenvalues from the plantar flexors were larger in young than in old muscle. For AT, this trend was only observed for λ1 and λ2. Although nonsignificant, λ3 was larger in old than in young AT. In contrast to the eigenvalues, FA was only significant in AT, with more anisotropy being observed in young than in old participants (Figure 1D).
Negligible differences in eigenvalues were observed when comparing groups by BMI (data not shown). Only λ2 and λ3 in MG were found to be significantly larger in participants designated Normal versus Over. In contrast, a significant increase in FA for MG was observed in participants designated Over versus Normal (data not shown).
Linear Regression
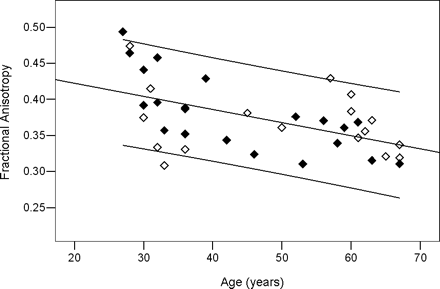
Presented in Table 1 are the significant fits of the DTMRI parameters to age for each muscle group. In general, the DTMRI parameters had an inverse relationship to age, represented by a negative slope. Only λ3 in AT produced a positive slope. Strong relationships to age were observed for λ1 and λ2 in the plantar flexors. In contrast, AT generated relationships of λ3 and FA to age. Linear trends of DTMRI parameters to height, weight, and BMI were not as prevalent as to age (data not shown). Nonetheless, significant fits were observed for MG, which included λ1 and λ2 to height (respectively, r = 0.373, p =.021, slope = 0.007 and r = 0.328, p =.045, slope = 0.005) and λ3 to BMI (r = 0.365, p =.024, slope = −0.12). When using the constants from Table 1, FA of AT declined by 20% from 27 years to 67 years of age. This parameter showed the largest change with age. A plot of the FA data and fit with age, including 95% confidence levels and designation of data by BMI group, are presented in Figure 2. The smallest was λ1 of SOL, with only a 7% reduction. In general, linear fits of the DTMRI parameters to age for the individual BMI groups produced significant results similar to what were observed from the entire population.
Results from the multiple regressions of the DTMRI parameters to age and anthropometric measures are presented in Table 2. A similar outcome to the previous analysis was observed (Table 1). From all the significant fits in Table 1, only λ3 in MG did not produce a significant result with age (p =.064). Relatively little change in the slope of the fits with respect to age was noticed between the multiple and simple regressions. This implies a strong relationship of the DTMRI parameters to age that is independent of the anthropometric measures. None of the anthropometric measures produced significant results when controlling for age.
Discussion
In this study we demonstrated that water diffusivity in skeletal muscle of the human calf varies as a function of age. Many of the DTMRI parameters were affected by age, whereas anthropometric measures had a negligible affect. We observed a significant reduction in the eigenvalues in older plantar flexors, but no change in FA. Conversely, FA was statistically lower in older AT with no large difference in the eigenvalues with age.
Structural changes within muscle tissue are presumed to be the main cause for the age-related variations reported in the eigenvalues of the plantar flexors. As observed in Figure 1, A–C, λ1, λ2, and λ3 varied between groups by roughly the same amount (SOL: −4.5%, −4.0%, and −5.2%, respectively; LG: −5.9%, −7.6%, and −7.0%, respectively; MG: −9.4%, −7.2%, and −8.1%, respectively). These results generated a similar FA between groups (SOL: 0.9%; LG: 2.4%; MG: −3.3%). A possible explanation for these observations may be the reduction of fiber size due to disuse atrophy. When healthy muscles are unused, muscle fibers shrink in size but may maintain their fiber type (44). It has also been shown by Lexell and colleagues (45) that age does not effect fiber-type composition. Therefore, a reduction in fiber size would simply confine a molecule to a more restricted space resulting in a smaller diffusion coefficient, i.e., eigenvalues. Our finding in the plantar flexors suggests that the geometry and structures of the fibers remain intact; the fibers in older individuals are simply smaller, which may be due to a more sedentary lifestyle.
The structural changes that occurred in AT due to aging differed from what was observed in the plantar flexors. Separating participants by the mean age of the population produced eigenvalues that were comparable between young and old AT. The principal eigenvalue in young AT was −2.9% larger than in old AT. In contrast, the second and third eigenvalues were 1.2% and 6.7%, respectively, smaller in young than in old AT. If the criterion for group selection were set at ages closer to the extremes, such as Young < 40 and Old > 59 (Young: 32 ± 0.8 n = 17; Old: 63 ± 0.8 n = 11), older AT would have generated a significantly larger λ3 than younger AT (0.91 ± 0.03 × 10−3 mm2/s vs 0.82 ± 0.02 × 10−3 mm2/s; p =.02). All previous results would have remained the same with this new selection criterion. Regardless of group cutoff, the relatively small difference in λ1 and λ2, and the large variation in λ3 resulted in a −9.6% difference in the mean FA of AT between Old and Young participants. FA dependence on aging suggests nonuniform alterations in AT muscle fiber structure. These observations imply that the structural changes that occurred in the muscle fibers of AT are inconsistent with those of the plantar flexors.
Differences in the muscular response of AT and the plantar flexors (MG and LG) to aging have been reported by Simoneau and colleagues (37). They showed that muscle torque decreases as a result of age in the plantar flexors but not in AT. Mechanical twitch in AT was also found to be preserved in the older population, which is in contrast to the plantar flexors. They state that the loss of muscular performance with age in the plantar flexors is linked to the atrophy phenomena at the peripheral level (37). Our observations suggest that some significant changes do occur in all muscle groups with aging. However, the extent of these changes differ between AT and the plantar flexors, which is in agreement with the observations of Simoneau and colleagues.
In general, DTMRI parameters decreased with increasing age. Similar trends were observed in the literature for muscle strength and power. Samson and colleagues (46) showed through simple linear and multiple regressions that age was the predominant factor in muscle performance (46). Nevertheless, muscle strength visibly had a dependence on height and weight in addition to age. Up to 50% of some performance measures were associated with height and weight. In contrast, our DTMRI parameters were independent of height, weight, and BMI. One inconsistency (λ3 in AT) was observed in our data. This parameter for this muscle generated the only positive slope for a statistical fit. It is worth noting that the magnitude of the slope was similar to that of the slopes of other statistical fits, just in the opposite direction. Although the fit is not shown graphically, λ3 in old AT was shown in Figure 1C to be larger than that in young AT. This finding shows the positive trend in λ3 with age. The factor that contributes to this result is unknown. Nevertheless, the effects of aging on AT differ significantly from those on the plantar flexors.
Some additional age-related alterations in muscle that would have a direct effect on our diffusion measurements include capillary density, peripheral venous pressure, and fiber type shifts. A loss in capillary density or peripheral venous pressure with age may alter muscle blood perfusion, thus adversely effecting our diffusion measurements. It has been reported in the literature that both capillary density (capillaries per mm2) (18,47) and peripheral venous pressure (48), in persons of ages similar to those in our study, does not change significantly with age. Conversely, literature observations of changing fiber type composition with age are mixed (18,47). Nevertheless, subtle changes in capillary density, venous pressure, or fiber type could effect measurements acquired by DTMRI. The contribution of perfusion on diffusion measurements can be reduced by using higher b values at a consequence of poorer signal-to-noise (49). In contrast, changes in fiber type composition would have a direct effect on DTMRI measurements due to alterations in subcellular barriers in the muscle, such as the sarcoplasmic reticulum and mitochondria (50). These age- and/or lifestyle-related changes highlight the difficulty in relating our results to any specific structural alteration in skeletal muscle.
The inclusion of athletes in our study warrants further attention. The athletes were included to fill the 50–60 year gap in our total study population. Due to the effects of exercise on skeletal muscle (51), this decision may have adversely affected our results. To determine their contribution to our observations, we had excluded the six participants from the population and repeated the analysis. Removal of the athletes resulted in a shift in the mean age of our population (44.6 ± 14.5 years) and a reduction in the number of Normal participants (n = 14). These changes had a negligible effect on our results and no effect on our final conclusion. The most notable difference was on the multivariate regression analysis, where the removal of the athletes resulted in a λ3 that had a significant relationship to age in LG and MG. Although muscle structure is highly sensitive to lifestyle and exercise, the small number of men participating in marathon training did not alter our results or conclusion.
We have demonstrated that parameters acquired by DTMRI are sensitive to age-related changes in skeletal muscle. In our previous work, we had speculated that λ2 may be more sensitive to age-related changes than the other eigenvalues (35). Our observations showed no such trend for the age range studied. Due to the complex structural hierarchy in muscle, it is difficult to decipher the exact factors that contribute to our observations. In addition, age-related alterations in fiber structure such as deformation and loss of muscle fibers, an increase of noncontractile mass (such as intramuscular fat), capillary network, and possibly fiber type shifts may have influenced our DTMRI results. Finally, lifestyle will also have a significant effect. Further research over a larger age range with other MRI and histological staining techniques are required in addition to DTMRI to better characterize how the eigenvalues and FA are affected in aging muscle.
Decision Editor: Luigi Ferrucci, MD, PhD
Box plots of the principal eigenvalues (λ1; A) second eigenvalues (λ2; B), third eigenvalues (λ3; C), and fractional anisotropy (FA; D) for all muscles in both age groups. Along the horizontal axis are the individual muscles: anterior tibialis (AT), lateral gastrocnemius (LG), medial gastrocnemius (MG), and soleus (SOL). Statistical significance is set at *p <.05 and **p <.01
Linear fit of the principal eigenvalue (λ1) with age in the soleus. This is the most significant fit for the soleus as shown in Table 2. Black diamonds: body mass index (BMI) < 25 kg/cm2; white diamonds: BMI ≥25 kg/cm2
Regression Line Constants, Pearson Correlation Coefficients (r), and p Values for λ1, λ2, λ3, and Fractional Anisotropy With Age.
| Parameters . | Slope . | y Intercept . | r . | p Value . | ||||
|---|---|---|---|---|---|---|---|---|
| SOL | ||||||||
| λ1 | −0.004 | 2.25 | −0.500 | .001 | ||||
| λ2 | −0.003 | 1.74 | −0.435 | .006 | ||||
| λ3 | −0.003 | 1.33 | −0.429 | .007 | ||||
| LG | ||||||||
| λ1 | −0.005 | 2.35 | −0.403 | .012 | ||||
| λ2 | −0.005 | 1.77 | −0.482 | .002 | ||||
| MG | ||||||||
| λ1 | −0.007 | 2.37 | −0.669 | <.0001 | ||||
| λ2 | −0.004 | 1.74 | −0.463 | .003 | ||||
| λ3 | −0.003 | 1.33 | −0.407 | .011 | ||||
| AT | ||||||||
| λ3 | 0.003 | 0.71 | 0.427 | .008 | ||||
| FA | −0.002 | 0.46 | −0.528 | .001 | ||||
| Parameters . | Slope . | y Intercept . | r . | p Value . | ||||
|---|---|---|---|---|---|---|---|---|
| SOL | ||||||||
| λ1 | −0.004 | 2.25 | −0.500 | .001 | ||||
| λ2 | −0.003 | 1.74 | −0.435 | .006 | ||||
| λ3 | −0.003 | 1.33 | −0.429 | .007 | ||||
| LG | ||||||||
| λ1 | −0.005 | 2.35 | −0.403 | .012 | ||||
| λ2 | −0.005 | 1.77 | −0.482 | .002 | ||||
| MG | ||||||||
| λ1 | −0.007 | 2.37 | −0.669 | <.0001 | ||||
| λ2 | −0.004 | 1.74 | −0.463 | .003 | ||||
| λ3 | −0.003 | 1.33 | −0.407 | .011 | ||||
| AT | ||||||||
| λ3 | 0.003 | 0.71 | 0.427 | .008 | ||||
| FA | −0.002 | 0.46 | −0.528 | .001 | ||||
Note: SOL = soleus; LG = lateral gastrocnemius; MG = medial gastrocnemius; AT = anterior tibialis.
Regression Line Constants, Pearson Correlation Coefficients (r), and p Values for λ1, λ2, λ3, and Fractional Anisotropy With Age.
| Parameters . | Slope . | y Intercept . | r . | p Value . | ||||
|---|---|---|---|---|---|---|---|---|
| SOL | ||||||||
| λ1 | −0.004 | 2.25 | −0.500 | .001 | ||||
| λ2 | −0.003 | 1.74 | −0.435 | .006 | ||||
| λ3 | −0.003 | 1.33 | −0.429 | .007 | ||||
| LG | ||||||||
| λ1 | −0.005 | 2.35 | −0.403 | .012 | ||||
| λ2 | −0.005 | 1.77 | −0.482 | .002 | ||||
| MG | ||||||||
| λ1 | −0.007 | 2.37 | −0.669 | <.0001 | ||||
| λ2 | −0.004 | 1.74 | −0.463 | .003 | ||||
| λ3 | −0.003 | 1.33 | −0.407 | .011 | ||||
| AT | ||||||||
| λ3 | 0.003 | 0.71 | 0.427 | .008 | ||||
| FA | −0.002 | 0.46 | −0.528 | .001 | ||||
| Parameters . | Slope . | y Intercept . | r . | p Value . | ||||
|---|---|---|---|---|---|---|---|---|
| SOL | ||||||||
| λ1 | −0.004 | 2.25 | −0.500 | .001 | ||||
| λ2 | −0.003 | 1.74 | −0.435 | .006 | ||||
| λ3 | −0.003 | 1.33 | −0.429 | .007 | ||||
| LG | ||||||||
| λ1 | −0.005 | 2.35 | −0.403 | .012 | ||||
| λ2 | −0.005 | 1.77 | −0.482 | .002 | ||||
| MG | ||||||||
| λ1 | −0.007 | 2.37 | −0.669 | <.0001 | ||||
| λ2 | −0.004 | 1.74 | −0.463 | .003 | ||||
| λ3 | −0.003 | 1.33 | −0.407 | .011 | ||||
| AT | ||||||||
| λ3 | 0.003 | 0.71 | 0.427 | .008 | ||||
| FA | −0.002 | 0.46 | −0.528 | .001 | ||||
Note: SOL = soleus; LG = lateral gastrocnemius; MG = medial gastrocnemius; AT = anterior tibialis.
Effects of Aging on λ1, λ2, λ3, and Fractional Anisotropy Controlling for Weight, Height, and BMI.
| Parameters . | Age . | Weight . | Height . | BMI . | r . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SOL | ||||||||||
| λ1 | −0.004* | 0.001 | −0.001 | −0.002 | 0.503 | |||||
| λ2 | −0.003† | −0.007 | 0.007 | 0.023 | 0.475 | |||||
| λ3 | −0.003† | −0.009 | 0.009 | 0.027 | 0.443 | |||||
| LG | ||||||||||
| λ1 | −0.005† | 0.008 | −0.007 | −0.021 | 0.417 | |||||
| λ2 | −0.005* | 0.003 | −0.002 | −2.7 × 10−5 | 0.526 | |||||
| MG | ||||||||||
| λ1 | −0.007* | −0.014 | 0.014 | 0.033 | 0.699 | |||||
| λ2 | −0.003† | −0.009 | 0.011 | 0.022 | 0.511 | |||||
| AT | ||||||||||
| λ3 | 0.004* | 0.018 | −0.016 | −0.059 | 0.493 | |||||
| FA | −0.002* | −0.008 | 0.007 | 0.026 | 0.574 | |||||
| Parameters . | Age . | Weight . | Height . | BMI . | r . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SOL | ||||||||||
| λ1 | −0.004* | 0.001 | −0.001 | −0.002 | 0.503 | |||||
| λ2 | −0.003† | −0.007 | 0.007 | 0.023 | 0.475 | |||||
| λ3 | −0.003† | −0.009 | 0.009 | 0.027 | 0.443 | |||||
| LG | ||||||||||
| λ1 | −0.005† | 0.008 | −0.007 | −0.021 | 0.417 | |||||
| λ2 | −0.005* | 0.003 | −0.002 | −2.7 × 10−5 | 0.526 | |||||
| MG | ||||||||||
| λ1 | −0.007* | −0.014 | 0.014 | 0.033 | 0.699 | |||||
| λ2 | −0.003† | −0.009 | 0.011 | 0.022 | 0.511 | |||||
| AT | ||||||||||
| λ3 | 0.004* | 0.018 | −0.016 | −0.059 | 0.493 | |||||
| FA | −0.002* | −0.008 | 0.007 | 0.026 | 0.574 | |||||
Notes: Slopes generated for all independent variables and Pearson correlation coefficient (r) from multiple linear regression are presented.
*p <.01.
†p <.05.
SOL = soleus; LG = lateral gastrocnemius; MG = medial gastrocnemius; AT = anterior tibialis; BMI = body mass index.
Effects of Aging on λ1, λ2, λ3, and Fractional Anisotropy Controlling for Weight, Height, and BMI.
| Parameters . | Age . | Weight . | Height . | BMI . | r . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SOL | ||||||||||
| λ1 | −0.004* | 0.001 | −0.001 | −0.002 | 0.503 | |||||
| λ2 | −0.003† | −0.007 | 0.007 | 0.023 | 0.475 | |||||
| λ3 | −0.003† | −0.009 | 0.009 | 0.027 | 0.443 | |||||
| LG | ||||||||||
| λ1 | −0.005† | 0.008 | −0.007 | −0.021 | 0.417 | |||||
| λ2 | −0.005* | 0.003 | −0.002 | −2.7 × 10−5 | 0.526 | |||||
| MG | ||||||||||
| λ1 | −0.007* | −0.014 | 0.014 | 0.033 | 0.699 | |||||
| λ2 | −0.003† | −0.009 | 0.011 | 0.022 | 0.511 | |||||
| AT | ||||||||||
| λ3 | 0.004* | 0.018 | −0.016 | −0.059 | 0.493 | |||||
| FA | −0.002* | −0.008 | 0.007 | 0.026 | 0.574 | |||||
| Parameters . | Age . | Weight . | Height . | BMI . | r . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SOL | ||||||||||
| λ1 | −0.004* | 0.001 | −0.001 | −0.002 | 0.503 | |||||
| λ2 | −0.003† | −0.007 | 0.007 | 0.023 | 0.475 | |||||
| λ3 | −0.003† | −0.009 | 0.009 | 0.027 | 0.443 | |||||
| LG | ||||||||||
| λ1 | −0.005† | 0.008 | −0.007 | −0.021 | 0.417 | |||||
| λ2 | −0.005* | 0.003 | −0.002 | −2.7 × 10−5 | 0.526 | |||||
| MG | ||||||||||
| λ1 | −0.007* | −0.014 | 0.014 | 0.033 | 0.699 | |||||
| λ2 | −0.003† | −0.009 | 0.011 | 0.022 | 0.511 | |||||
| AT | ||||||||||
| λ3 | 0.004* | 0.018 | −0.016 | −0.059 | 0.493 | |||||
| FA | −0.002* | −0.008 | 0.007 | 0.026 | 0.574 | |||||
Notes: Slopes generated for all independent variables and Pearson correlation coefficient (r) from multiple linear regression are presented.
*p <.01.
†p <.05.
SOL = soleus; LG = lateral gastrocnemius; MG = medial gastrocnemius; AT = anterior tibialis; BMI = body mass index.
This work was supported by a grant from the Ministry for Science and Research of the state of North Rhine-Westphalia, Germany.
References
Doherty TJ. The influence of aging and sex on skeletal muscle mass and strength.
Larsson L, Ramamurthy B. Aging-related changes in skeletal muscle. Mechanisms and interventions.
Nikolic M, Bajek S, Bobinac D, Vranic TS, Jerkovic R. Aging of human skeletal muscles.
Roubenoff R. Sarcopenia: effects on body composition and function.
Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women.
Marcell TJ. Sarcopenia: causes, consequences, and preventions.
Sowers MR, Crutchfield M, Richards K, et al. Sarcopenia is related to physical functioning and leg strength in middle-aged women.
Goldspink G. Age-related muscle loss and progressive dysfunction in mechanosensitive growth factor signaling.
Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr.
Larsson L, Yu F, Hook P, Ramamurthy B, Marx JO, Pircher P. Effects of aging on regulation of muscle contraction at the motor unit, muscle cell, and molecular levels.
Owino V, Yang SY, Goldspink G. Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload.
Pahor M, Kritchevsky S. Research hypotheses on muscle wasting, aging, loss of function and disability.
Andersen JL. Muscle fibre type adaptation in the elderly human muscle.
Chan KM, Doherty TJ, Brown WF. Contractile properties of human motor units in health, aging, and disease.
Frontera WR, Hughes VA, Krivickas LS, Roubenoff R. Contractile properties of aging skeletal muscle.
Lexell J. Human aging, muscle mass, and fiber type composition.
Larsson L. Motor units: remodeling in aged animals.
Luff AR. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units.
Morse CI, Thom JM, Birch KM, Narici MV. Changes in triceps surae muscle architecture with sarcopenia.
Kent-Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men.
Sinha U, Yao L. In vivo diffusion tensor imaging of human calf muscle.
Jones DK, Simmons A, Williams SC, Horsfield MA. Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI.
Hsu EW, Muzikant AL, Matulevicius SA, Penland RC, Henriquez CS. Magnetic resonance myocardial fiber-orientation mapping with direct histological correlation.
Clark CA, Werring DJ, Miller DH. Diffusion imaging of the spinal cord in vivo: estimation of the principal diffusivities and application to multiple sclerosis.
Ries M, Jones RA, Basseau F, Moonen CT, Grenier N. Diffusion tensor MRI of the human kidney.
Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications.
Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging.
Damon BM, Ding Z, Anderson AW, Freyer AS, Gore JC. Validation of diffusion tensor MRI-based muscle fiber tracking.
Van Donkelaar CC, Kretzers LJ, Bovendeerd PH, et al. Diffusion tensor imaging in biomechanical studies of skeletal muscle function.
Galban CJ, Maderwald S, Uffmann K, de Greiff A, Ladd ME. Diffusive sensitivity to muscle architecture: a magnetic resonance diffusion tensor imaging study of the human calf.
Galban CJ, Maderwald S, Uffmann K, Ladd ME. A diffusion tensor imaging analysis of gender differences in water diffusivity within human skeletal muscle.
Simoneau E, Martin A, Van Hoecke J. Muscular performances at the ankle joint in young and elderly men.
Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups?
Madigan ML, Lloyd EM. Age and stepping limb performance differences during a single-step recovery from a forward fall.
Hoffman M, Schrader J, Applegate T, Koceja D. Unilateral postural control of the functionally dominant and nondominant extremities of healthy subjects.
Kim S, Chi-Fishman G, Barnett AS, Pierpaoli C. Dependence on diffusion time of apparent diffusion tensor of ex vivo calf tongue and heart.
Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images.
Berg HE, Larsson L, Tesch PA. Lower limb skeletal muscle function after 6 wk of bed rest.
Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men.
Samson MM, Meeuwsen IB, Crowe A, Dessens JA, Duursma SA, Verhaar HJ. Relationships between physical performance measures, age, height and body weight in healthy adults.
Harris BA. The influence of endurance and resistance exercise on muscle capillarization in the elderly: a review.
Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age.
Melhem ER, Itoh R, Jones L, Barker PB. Diffusion tensor MR imaging of the brain: effect of diffusion weighting on trace and anisotropy measurements.
Kinsey ST, Locke BR, Penke B, Moerland TS. Diffusional anisotropy is induced by subcellular barriers in skeletal muscle.